3rd level of atomic struture can hold how many electrons
What term is used for the electrons in the outermost shell or energy level. Neutral atom of the element above.

2 1 2 2 Atomic Structure Sl Youtube
The electrons are found at considerable distances from the nucleus in a series of levels called energy levels.
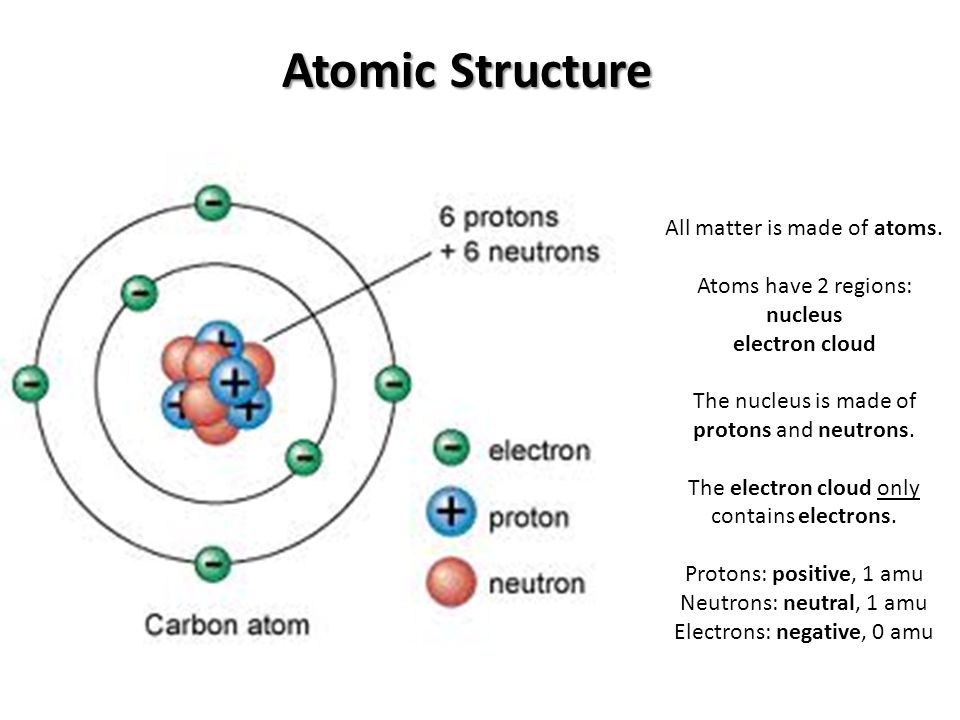
. Uranium has three common isotopes. Therefore the total number of shells 3. When we draw electrons we use up and down arrows.
Ar 1s 2 2s 2 2p 6 3s 2 3p 6 Sometimes you will see the notation. Otherwise they would have the same four quantum numbers which is forbidden. Third shell can also holds up to 8 electrons.
Using the chart below determine the average atomic mass of Uranium. Sub shells are known by letters s p d and f. A single orbital can hold a maximum of two electrons which must have opposing spins.
One electron is spin up m s 12 and the other would spin down m s -12. The negatively charged particles called electrons revolve around the centre of the nucleus. How many electrons can each level hold.
An orbital holds 2 electrons. Submit your answer with three decimal places. The 3rd energy level of an atom can hold how many electrons.
Scientists use two types of diagrams to show the electron configuration for atoms. So if an electron is paired up in a box one arrow is up and the second must be down. When you get past argon Ar at atomic number 18 you will start finding the d suborbitals in the transition elements.
The n 1 shell is filled with two electrons and three electrons will occupy the n 2 shell. It is written by the number of electrons in the 1st shell max 2 then a commer the number of electrons in the 2nd shell. 5 What are the Uses of Carbon.
The history of atomic structure and quantum mechanics dates back to the times of Democritus the man who first proposed that. How many p-orbitals are occupied in a K atom. Rule 1 Pauli Exclusion Principle An atomic orbital can hold a maximum of two electrons and these two electrons must have different spin states spin-up and spin-down.
Electronic structure is how many electrons each shell of an atom can hold. Ne3s 2 3p 6 which means to include everything that is in neon Ne 10 plus the stuff in the 3-level. Atoms and Atomic Structure.
How many atomic orbitals are there in the 4p sublevel. 1st 2 2nd 8 3rd 18 13. The table below shows its electron configuration as 3s 2 3p 6 remembering that p orbitals can hold up to six 6 electrons.
These rules are derived from the properties of the electron. In this video we cover the structure of atoms what are subatomic particles energy levels and stable and reactive atomsTranscript and notesAtomic structur. To find the atomic mass add the number of protons and neutrons.
How many atomic orbitals are there in a g subshell. Suborbital s can hold two and the other two are found in p. The periodic table is arranged by atomic mass.
The atomic number of calcium is 20. 2 How Many Electrons Can A Carbon Atom Share. An atom of boron atomic number 5 contains five electrons.
Because any s subshell can contain only two electrons the fifth electron must occupy the next energy level which will be a 2p orbital. Where Are the Electrons. 2 How many electrons would there be in a.
This model can be further refined by the concept of sub shells and orbitals. For atoms with atomic numbers less than about 20 the octet rule of electron addition and orbital filling applies. The following rules guide us on how to fill atomic orbitals with electrons.
3 What is Carbon. The first level nearest the nucleus will only hold 2 electrons the second holds 8 and the third also seems to be full when it has 8 electrons. 6 How many electrons does a carbon atom have in its outer energy level.
A neutral atom of an element has 2 electrons in the first energy level 8 in the second energy level and 8 in the third energy level. The further a shell is from the nucleus the higher the energy level. Each energy level can only hold a certain number of electrons.
The third orbit can fill up to 18 electrons and it will accommodate left electrons of the element. Electron configuration means the arrangement of. 4 What are the Properties of Carbon.
Atomic structure refers to the structure of an atom comprising a nucleus centre in which the protons positively charged and neutrons neutral are present. A the atomic number of the element. A 6 b 12 c 6011 d 18.
What term is used for the electrons in the outermost shell or energy level. The s sub shell can contain 2 electrons p 6 d 10 and f 14. 1 How many electrons are in carbon.
Follow your teachers directions to complete the diagrams. Why are s orbitals non directional. Each type of shell has a different type of orbital.
How many electrons can each level hold. 1st 2 2nd 8 3rd 18 13. Electronic configuration of Argon 2 8 8.
Electrons occupy negative charge clouds called orbitals each orbital can hold only 2 electrons. Most of the mass of an element is in the electron shell. 8 electrons In the periodic table there are 2 electrons in period 1 while both periods 2 and 3 have 8 electrons in the filled level.
Silicon only has four electrons in the third shell. So there are 20 protons and 20. Here is an animation of how the orbitals would look as you build out from the 1s.
Sulfur Atomic 16 Atomic Mass 32 Protons 16. To find neutrons subtract the number of protons from the atomic mass. This information does not necessarily tell us.
Uranium is used in nuclear reactors and is a rare element on earth. Weve been telling you that electrons reside in specific shells or move in specific patterns in suborbitals. Its actual electron configuration is.
7 How many electrons does one atom of carbon share to complete its valence shell. A Bohr model shows the atomic structure of an atom. If ℓ is the angular quantum number of subshell then maximum electrons it can hold is 22 ℓ 1.
Chem4kids Com Potassium Orbital And Bonding Info

Atomic Structure Of Matter Energy Levels Electronic Distribution Chemical Activity Science Online
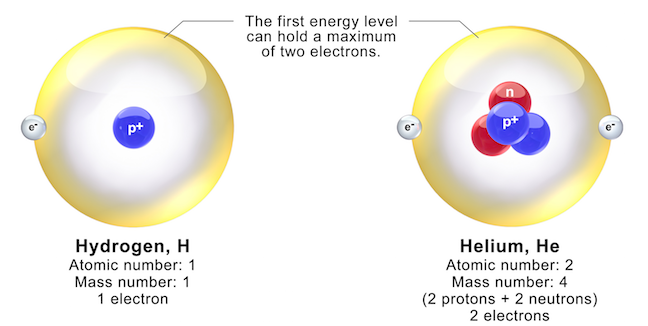
5 1 Atoms Physical Geology First University Of Saskatchewan Edition
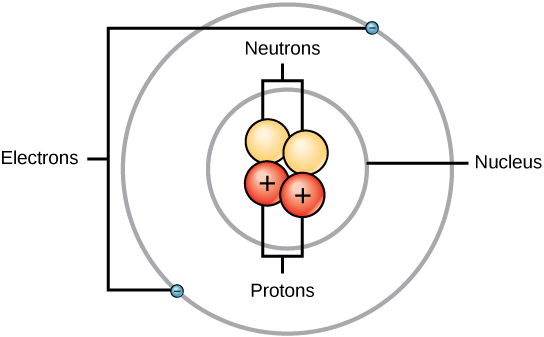
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
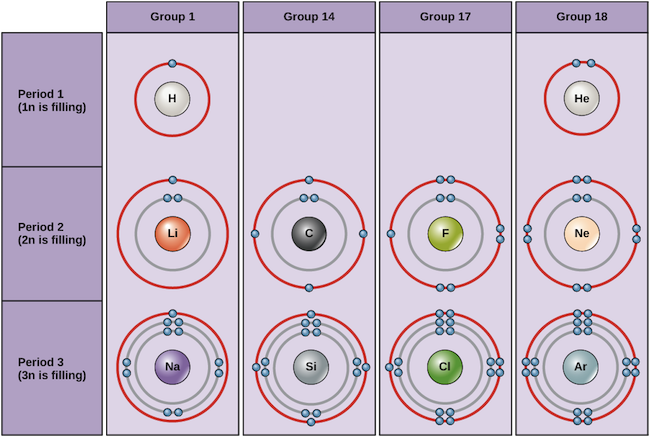
The Periodic Table Electron Shells And Orbitals Article Khan Academy

Electron Configuration Boundless Chemistry
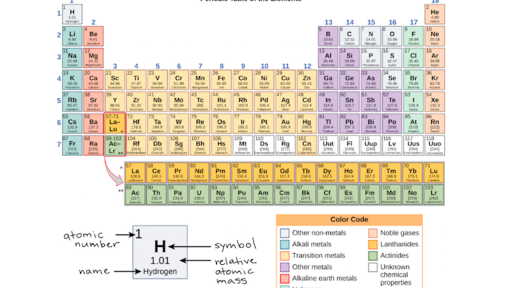
The Periodic Table Electron Shells And Orbitals Article Khan Academy
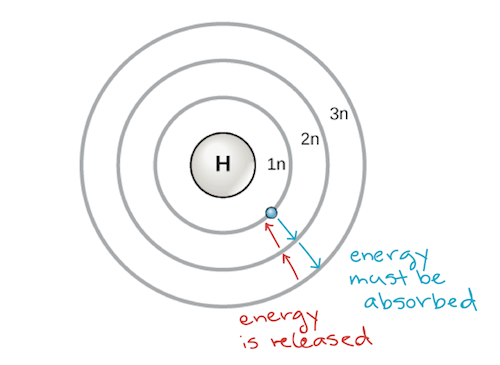
The Periodic Table Electron Shells And Orbitals Article Khan Academy
0 Response to "3rd level of atomic struture can hold how many electrons"
Post a Comment